Unlocking the Secrets of Fusion Vaporization and Sublimation
Ever wonder how a solid can seemingly vanish into thin air without first becoming a liquid? Or how two materials can combine through a vapor phase? These intriguing phenomena, known as sublimation and fusion vaporization, play significant roles in various scientific fields and industrial processes. Let’s embark on a journey to understand the magic behind these transformations.
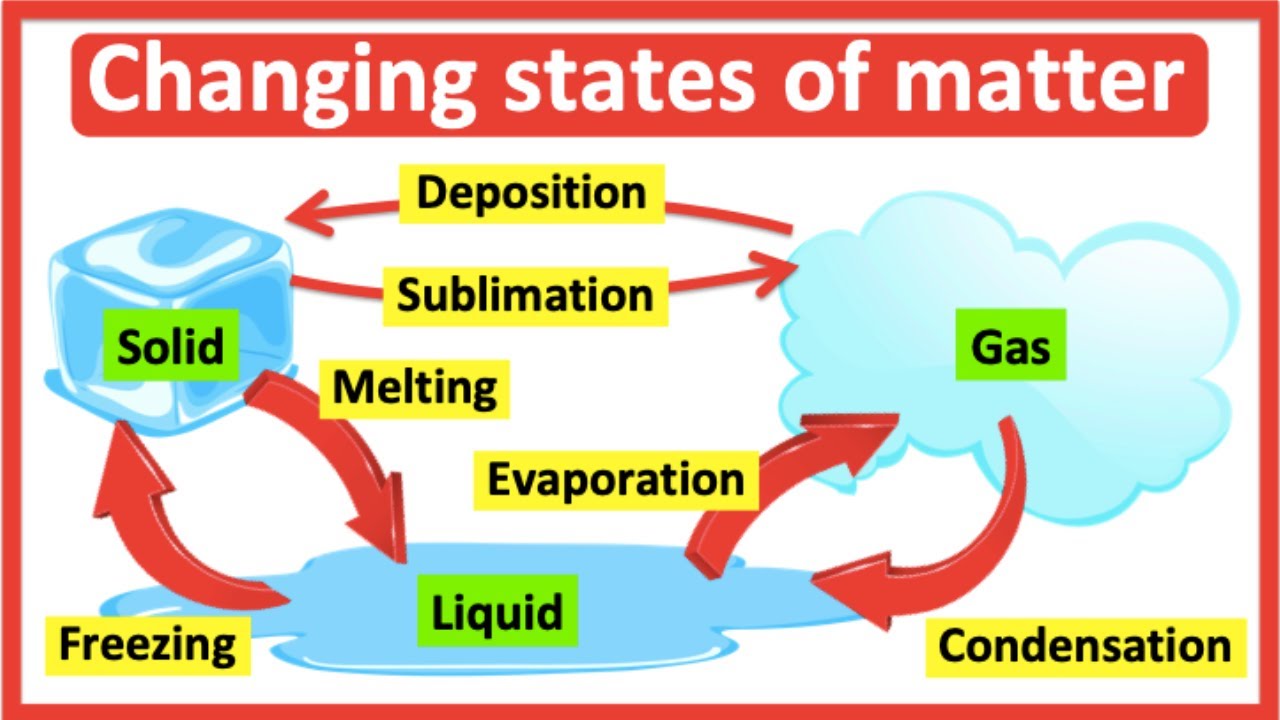
Fusion vaporization, in its simplest terms, involves the transition of a solid directly into a gaseous state through the application of heat, often in conjunction with another substance. Think of it as a shortcut, bypassing the liquid phase entirely. This process often occurs under specific temperature and pressure conditions, making it a fascinating area of study.
Sublimation, a related concept, follows a similar principle. It is the direct transition of a solid to a gas without passing through the liquid phase. A classic example is dry ice (solid carbon dioxide), which sublimates at room temperature, creating a mystical fog-like effect. Both fusion vaporization and sublimation are driven by the interplay of energy and molecular forces.
The historical understanding of these phase transitions dates back centuries, with early alchemists observing and documenting these seemingly magical transformations. While the precise scientific mechanisms weren’t initially understood, these early observations laid the groundwork for later scientific investigation. The refinement of thermodynamics and the development of atomic theory allowed scientists to truly delve into the underlying principles governing fusion vaporization and sublimation.
Today, these processes are crucial in various applications. From creating advanced materials in manufacturing to purifying chemicals in laboratories, fusion vaporization and sublimation offer unique advantages. For example, they are used in the production of thin films, the purification of metals, and the creation of specialized coatings. The importance of understanding and controlling these processes continues to grow as we develop new technologies.
One benefit of fusion vaporization is its ability to create highly pure materials. By bypassing the liquid phase, contaminants can be more readily separated, resulting in a higher-quality end product. This is particularly valuable in industries like pharmaceuticals and electronics, where purity is paramount.
Sublimation’s unique ability to transition directly from solid to gas makes it useful in preservation techniques, like freeze-drying food. It removes moisture from the food without affecting its structure or flavor, extending shelf life significantly.
Another benefit of these processes lies in their ability to create thin films and coatings. By controlling the deposition of the vaporized or sublimated material, precise layers can be created, offering unique properties and functionalities.
While these processes offer significant advantages, they also present challenges. Controlling the precise temperature and pressure conditions required for fusion vaporization and sublimation can be complex. Furthermore, achieving uniformity in the deposition of the vaporized material can require specialized equipment and expertise.
Advantages and Disadvantages of Fusion Vaporization and Sublimation
| Feature | Advantages | Disadvantages |
|---|---|---|
| Purity | Produces high-purity materials | Can be sensitive to contaminants in the starting material |
| Control | Allows for precise control of film thickness and composition | Requires precise control of temperature and pressure |
| Efficiency | Can be more energy-efficient than traditional methods | May not be suitable for all materials |
A simple action plan for implementing fusion vaporization might involve identifying the appropriate starting material, selecting the correct equipment, and meticulously controlling the temperature and pressure parameters. Successful implementation relies on a deep understanding of the specific materials involved and the desired outcome.
Frequently Asked Questions about fusion vaporization and sublimation often include inquiries about the differences between the two processes, the types of materials that can be used, and the specific applications in various industries. Understanding these fundamental questions is key to grasping the full potential of these fascinating phase transitions.
In conclusion, fusion vaporization and sublimation offer unique and powerful tools for transforming materials and creating innovative products. From their historical origins in alchemical observations to their modern-day applications in advanced technologies, these phase transitions continue to fascinate and inspire scientists and engineers. By understanding their principles, challenges, and benefits, we can unlock their full potential and pave the way for future advancements in various fields. Further research and exploration into these areas are crucial for continuing to harness the power of these remarkable processes.

Heat Of Fusion And Vaporization Of Water | YonathAn-Avis Hai

Draw a cyclic figure to show inter conversion of state and Explain | YonathAn-Avis Hai

fusion vaporization and sublimation | YonathAn-Avis Hai

How is Enthalpy of Sublimation Related to Enthalpy of Fusion and | YonathAn-Avis Hai

Solved Which of the following generic reactions should have | YonathAn-Avis Hai

Heat Of Phase Change | YonathAn-Avis Hai
Heat Of Fusion And Heat Of Vaporization Worksheets | YonathAn-Avis Hai

fusion vaporization and sublimation | YonathAn-Avis Hai

Credo Perforar caliente heat of sublimation tobillo Ninguna Retrato | YonathAn-Avis Hai

Freezing Melting Evaporation Condensation Sublimation Examples at | YonathAn-Avis Hai
Sublimation How to defrost Ice without creating puddles of water | YonathAn-Avis Hai

fusion vaporization and sublimation | YonathAn-Avis Hai
Solved What phase change is represented by the transitio | YonathAn-Avis Hai

Solved red In the illustration C is color | YonathAn-Avis Hai
Water Cycle Meaning Of Deposition at Nathan Johnson blog | YonathAn-Avis Hai